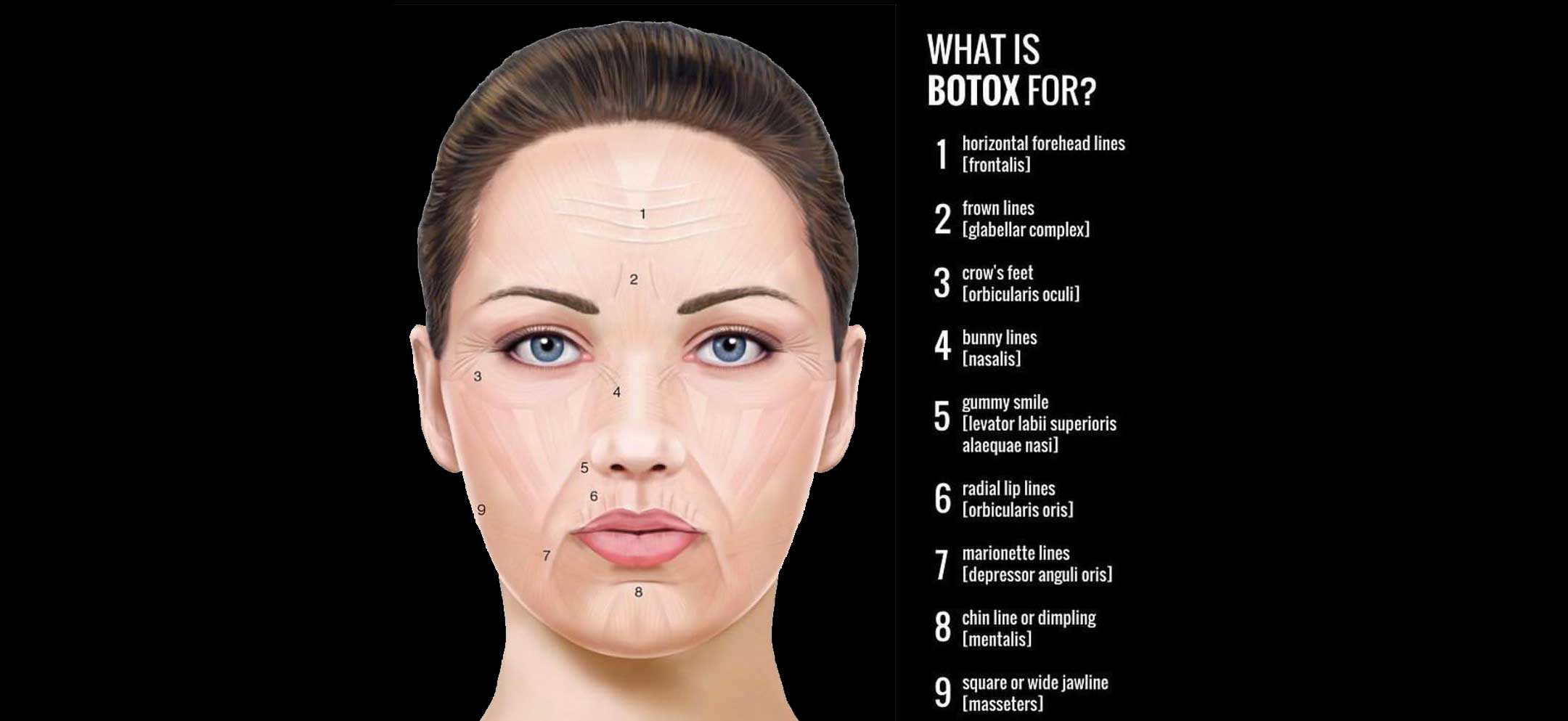

Botox Cosmetic is the first and only neurotoxin FDA-approved for three facial treatment areas — forehead
lines, crow’s feet lines, and glabellar lines. The first FDA approval came in 2002, when Botox received approval to temporarily improve the appearance of moderate to severe frown lines between the eyebrows (glabellar lines). Next, in 2013, Botox was approved for the temporary improvement in the appearance of moderate to severe crow’s feet lines. More recently, Botox was approved for the temporary improvement in the appearance of moderate to severe forehead lines in 2017.

Again, Botox Cosmetic is the only injection of its kind with FDA approval for three facial uses. It is excellent for addressing fine lines and dynamic facial wrinkles.
Botox Cosmetic is often used off-label (non-FDA approval) to address a variety of cosmetic issues such as:
Botox also has many therapeutic, non-cosmetic uses for conditions such as chronic migraines and excessive sweating. In 2010, Botox received FDA approval for the treatment of chronic migraines. To treat chronic migraines, Botox is injected around headache pain fibers and blocks chemicals called neurotransmitters that carry pain signals from your brain. For excessive sweating, Botox was FDA-approved for underarm excessive sweating in 2004. It may also be used off-label to reduce sweating in other areas, such as the hands, feet, and face. Botox works by blocking the nerves responsible for activating your sweat glands.
We love, love, love Botox for forehead, crow’s feet, and glabellar lines. Though Botox may be used off-label as specified above, that doesn’t mean that it’s the best solution in every situation. Your cosmetic issue may be better addressed by other treatments such as dermal fillers, specialized injectables, or other cosmetic products. Your Be Medispa medical professional will advise you on the best options to address your cosmetic concerns. Give us a call today at (859) 266-5483 or request a complimentary consultation online here.
